Effective Ways to Find Protons, Neutrons, and Electrons in 2025
Understanding the basic atomic particles—**protons**, **neutrons**, and **electrons**—is essential in the study of **atomic structure** and chemistry. This guide will explore effective techniques for finding protons, neutrons, and electrons in an atom, the significance of atomic numbers, mass numbers, and more, all in the context of advancements in chemistry as we look into 2025. Illustrated below are the foundational principles that will enhance your comprehension of the atomic universe.
Understanding Atomic Structure
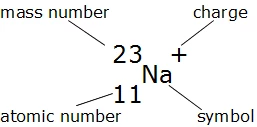
The atomic structure serves as the foundation for understanding how **atoms and elements** compose matter. Atoms consist of a nucleus, where **protons** and **neutrons** reside, surrounded by a cloud of electrons. The **atomic number**—the number of protons present in an atom—uniquely identifies each chemical element on the **periodic table**.
The Role of Protons
Protons play a vital role in defining the identity of an atom. Each chemical element has a unique number of protons, forming the basis of its **atomic number**. For example, carbon has six protons, indicated by its atomic number of 6. **Identifying protons** involves understanding where they can be found within the atomic nucleus, contributing to both atomic stability and chemical behavior. The interrelationship between **protons and neutrons** also adds complexity, particularly in **nuclear stability**, as their pairing influences the **mass number** of the element.
Neutrons and Their Importance
**Neutrons** are critical for the stability of the nucleus. They are neutral particles that counterbalance the positive charge of protons, helping to hold the nucleus together. The number of neutrons can vary even within atoms of the same element, leading to different isotopes. Understanding **neutrons and atomic weight** is important when analyzing atomic composition, as the mass number is the sum of protons and neutrons. Notably, the success of methods used to **determine neutrons** affects fields such as nuclear medicine and stability calculations in material sciences.
Calculating Electrons
Electrons are negatively charged subatomic particles that occupy regions around the nucleus called electron shells. The total number of electrons in a neutral atom usually matches the number of protons, reflecting the atomic number. Knowing **how to calculate electrons** is essential in various chemical reactions, where electron behavior significantly impacts bonding and reaction dynamics. For instance, understanding how to apply the **electron configuration** helps predict how atoms engage in chemical bonding, thereby enriching the study of chemistry.
Methods of Finding Protons, Neutrons, and Electrons
Finding the counts of protons, neutrons, and electrons in an atom involves a mix of theoretical knowledge and practical applications. This section presents several methods to accurately obtain these counts.
Identifying Protons via Atomic Number
The easiest method to find **protons** in an atom is simply by determining its **atomic number** from the periodic table. For each element listed, the atomic number directly corresponds to the number of protons. For instance, an atomic number of 8 indicates there are 8 protons, which defines oxygen as an element. This reliable method is fundamental in **basic chemistry concepts** and serves as an entry point into atomic study.
Determining Neutrons Using Mass Number
To calculate the number of **neutrons**, one can use the mass number (total number of protons and neutrons). The formula to find neutrons is straightforward: subtract the atomic number from the mass number. For example, if an isotope of carbon has a mass number of 14, the calculation would be 14 (mass number) – 6 (atomic number) = 8, indicating there are 8 neutrons. This technique for **determining neutrons** is vital in understanding isotopes and their applications in science and medicine.
Calculating Electrons in Ions
When identifying **electrons** in an atom, one must consider if the atom is neutral or ionic. For neutral atoms, count of electrons equals that of protons. Conversely, for ions, the charge will dictate the electron count. For example, a sodium ion (Na+) has 11 protons but only 10 electrons. Thus, learning **how to find electrons** in both neutral and charged states is essential for areas like where elements engage in reactions and form compounds.
Historical Perspectives on Atomic Research
Throughout the centuries, research on the structure of atoms has evolved remarkably. Historical theories provide a framework for modern understanding and reveal the complex developments of atomic science.
Rutherford’s Experiment
Rutherford’s gold foil experiment was pivotal in unveiling the structure of the atom. It demonstrated that most of an atom’s mass and its positive charge are concentrated in a small nucleus, a significant divergence from the previously accepted plum pudding model. This experiment helped identify the existence of protons and set the stage for future exploration into neutrons, quickly transforming our understanding of atomic theory.
The Bohr Model and Quantum Mechanics
Developed in the early 20th century, the Bohr model enhanced the foundational concepts laid by Rutherford. By introducing quantized electron orbits, Bohr’s model aligned with the principles of **quantum mechanics** while explaining the spectral lines observed in gases. The Bohr model not only refined the understanding of **electron configurations** but also contributed to defining the energy levels within atoms as seen in the periodic table.
Modern Atomic Theory
Today, atomic theory continues to evolve through extensive research in fields such as quantum mechanics and particle physics. New discoveries reveal the subatomic structure and interactions of particles, enhancing the application of atomic theory in both chemistry and material science. Expanding our understanding of **basic atomic particles** through methods like **isotopic analysis** and particle collision experiments leads to exciting developments and deepens the integration of chemistry with physics.
Key Takeaways
- Understanding **atomic structure** is crucial for recognizing the roles of protons, neutrons, and electrons.
- **Protons** and **atomic numbers** define elemental identity, while **neutrons** impact **nuclear stability** and isotopic characteristics.
- Calculating **electrons** covers both neutral atoms and ions, showcasing their role in chemical bonding.
- Historical experiments like Rutherford’s and advances in the **Bohr model** laid the groundwork for modern atomic theory.
- Continuing research enhances our understanding of **chemical elements** and their interactions.
FAQ
1. What is the significance of atomic number in finding protons?
The **atomic number** indicates the number of protons in an atom, which uniquely identifies the element. Without this number, one cannot accurately ascertain the **identity of protons** within an atom, making it essential in the study of elements.
2. How are electrons calculated in ions?
To determine **electrons** in ions, one must consider the charge. In a neutral atom, the electron count equals the number of protons. However, for positive ions like Na+, the count is reduced by the charge, whereas it is increased for negative ions like Cl–.
3. Can you explain the role of neutrons in isotopes?
**Neutrons** are crucial in defining isotopes, which have the same number of protons but a different number of neutrons. This variance impacts their mass and stability, directly relating to the concept of **neutrons and atomic weight**, which are pivotal in many scientific applications.
4. What discoveries led to modern atomic theory?
Modern atomic theory greatly benefited from historical experiments such as **Rutherford’s experiment**, the selection of models like the **Bohr model**, and advancements in **quantum mechanics**. These discoveries collectively shaped our understanding of subatomic particles and their interactions within the atom.
5. How does the electron configuration affect chemical behavior?
The **electron configuration** of an atom defines how electrons are arranged in the shells around the nucleus, influencing its chemical properties and reactions. Atoms will behave differently based on their **electron shells**, ultimately determining their bonding capabilities.